Detailed information relating to LST Heavy Liquid.
Density & viscosity
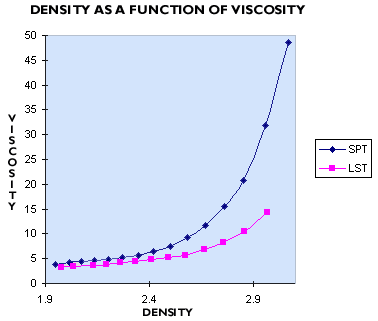
In the high density region where both LST Heavy Liquid and SPT are used as heavy liquids (2.8 to 2.9 g/mL), it can be seen that the viscosity of SPT is considerably higher. At a density of 2.85 g/mL at 25oC, SPT has a viscosity of 20 cP, whereas LST has a viscosity of 10 cP. LST has similar viscosities to TBE and so mineral settling times remain comparable. As the viscosity of SPT is twice that of LST and TBE, its settling rates for mineral samples are therefore twice as long. This means that in practice the times required for separation when using SPT become prohibitively long.
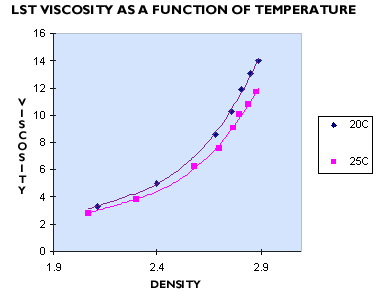
LST viscosities decrease slightly with an increase in temperature. Figure 2 shows a 1.5 cP drop in viscosity when the temperature is raised from twenty to twenty-five degrees centigrade. Faster density separations can therefore be achieved through an increase in solution temperature.

Physical characteristics
LST Heavy Liquid solids contain heteropolytungstates in the form of extremely soluble, colourless, hydrated crystals. Concentrated aqueous solutions of LST Heavy Liquid form colourless or pale yellow heavy liquids.
LST Heavy Liquid is normally used as an aqueous solution. However, the LST Heavy Liquid crystals are also highly soluble in acetone and alcohols, and can form dense liquids in these organic solvents. These provide a range of methods for the use and recycling of LST heavy liquids.
Solutions of LST Heavy Liquid have been found to be stable up to 140oC for at least two weeks. No decomposition, or loss of maximum density was observed during this time. This means that solutions of LST Heavy Liquid which have been diluted by washing etc. can easily be reconcentrated by heating to boiling when evaporating off excess water.

More about heavy liquids
LST Heavy Liquid solids contain heteropolytungstates in the form of extremely soluble, colourless, hydrated crystals. Concentrated aqueous solutions of LST Heavy Liquid form colourless or pale yellow heavy liquids.
LST Heavy Liquid is normally used as an aqueous solution. However, the LST Heavy Liquid crystals are also highly soluble in acetone and alcohols, and can form dense liquids in these organic solvents. These provide a range of methods for the use and recycling of LST heavy liquids.
Solutions of LST Heavy Liquid have been found to be stable up to 140oC for at least two weeks. No decomposition, or loss of maximum density was observed during this time. This means that solutions of LST which have been diluted by washing etc. can easily be reconcentrated by heating to boiling when evaporating off excess water.

Uses
Heavy liquids are dense fluids or solutions used to separate materials of different density through their buoyancy. Materials with a density greater than the heavy liquid will sink, while materials with a density less than the heavy liquid will float on the surface. In the analysis of mineral samples, separation based on mineral density is an important step in many sectors of the mining industry. On a plant scale the methods used include jigs, spirals, riffle tables and heavy medium separation. On a laboratory scale, heavy liquids are commonly used.
Laboratory heavy liquid separations result in two components, the heavy fraction (or sinks) and the light fraction (or floats). In industry it is usually the heavy fraction of a mineral suite which contains the valuable component, and which is separated from sand (silica) and other components having a density less than 2.7 g/mL. The solution density used for this type of separation is about 2.85 g/mL, nearly three times the density of water.
Another use of heavy liquids is in paleontology. Typically, these heavy liquid separations are conducted at a lower density (e.g. 2.2 g/mL) since the separation is not between minerals of different types, but between fossil bones and minerals.

Inorganic tungsten based heavy liquids: SPT, LMT and LST
Two older isopolytungstate substitutes for bromoform and TBE have been available for some time, and are known as sodium metatungstate or sodium polytungstate (SPT) and lithium metatungstate (LMT). The tungsten anion for SPT and LMT is [H2W12O40]6- . They belong to a class of structures known in the literature as the 'Keggin' species.
Both SPT and LMT are relatively non-toxic and generally inert, but are considerably more viscous than bromoform and TBE at high densities. At a density of 2.85 g/mL, where TBE has a viscosity of 10 cP and bromoform has a viscosity of 2 cP, SPT has a viscosity of over 20 cP. This high viscosity results in considerably longer settling and filtration times when using SPT. SPT also has poor thermal stability above 80oC, making recovery and concentration more difficult during the recycling of the heavy liquid.
A more thermally stable alternative of considerably lower viscosity is LST Heavy Liquid. LST Heavy Liquid can be used up to a density of 2.9 g/ml at room temperature, and up to a density of 3.5 g/ml at elevated temperatures.
Polyhedral representation of the 'Keggin' structure

Organic based heavy liquids
The organic heavy liquids form the "older" generation of heavy liquids. The two organic heavy liquids most used at present to effect mineral separations are bromoform (CHBr3) or TBE (1,1,2,2-tetrabromoethane; C2H2Br4). Bromoform has a density of 2.87 g/mL and TBE a density of 2.95 g/mL. Of these, bromoform has the lower viscosity (1.8 cP) but is considered more hazardous to work with because it has the higher vapour pressure (5.9 mm Hg at 25oC). TBE has a higher viscosity (9 cP) and a lower vapour pressure (0.02 mm Hg at 25oC).
Another organic heavy liquid, used when higher densities are required, is methylene iodide. Methylene iodide has a density of 3.31 g/mL, a vapour pressure of 1.2 mm Hg at 25oC, and a low viscosity of 2.6 cP
The disadvantage of the above heavy liquids is that they are highly toxic and require stringent conditions to minimise exposure to workers. The most widely used heavy liquids, bromoform and TBE, for example, are thought to be highly toxic. Both liquids can be absorbed through inhalation and ingestion and with bromoform, through the skin. TBE was found to be quite toxic when given in small, prolonged and repeated doses and so has a Worksafe Australia exposure standard of 1ppm (or 14 mg/m3) for a time-weighted average concentration. TBE is known to cause eye, skin and respiratory irritation and headaches, nausea and kidney and liver damage. TBE is also a suspected carcinogen.
Inhalation of bromoform vapours cause nose and throat irritation. It can also irritate the eyes and cause lachrymation. By analogy with the closely related substance chloroform, it could be suspected to cause liver and kidney damage. Animal tests suggest bromoform may be carcinogenic but as yet no human information is available. The 1991 Worksafe Australia TLV standard for bromoform is set at 0.5 ppm due to bromoform's irritant qualities and reported skin absorption.

![]()
